How to Sterilize and Maintain a Medical Balloon Catheter Properly?
Introduction
The medical balloon catheter is a critical device in interventional and surgical procedures, valued for its precision and reliability in vessel dilation, fluid management, and minimally invasive treatments. Despite its advanced engineering, the catheter’s effectiveness and safety rely heavily on proper sterilization and maintenance. Inadequate cleaning or improper storage may compromise device integrity, contamination risks or performance degradation.
Understanding the Structure and Sensitivity of a Medical Balloon Catheter
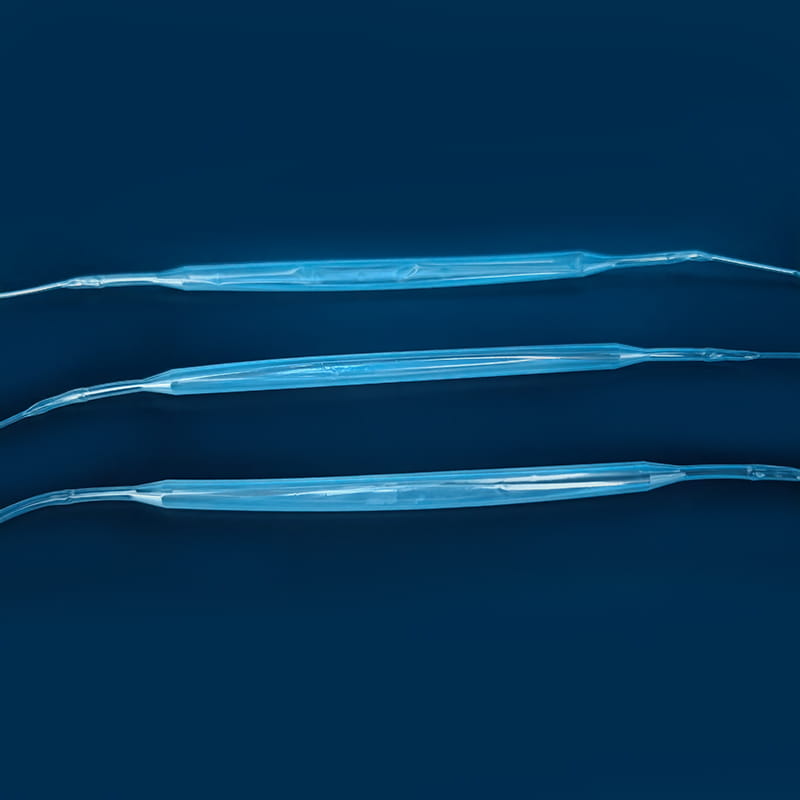
A medical balloon catheter is composed of three major components: the catheter shaft, the balloon segment, and the connector interface. Each part is made from precision-engineered materials such as polyethylene, polyurethane, or nylon, which provide flexibility, chemical resistance, and biocompatibility. However, these materials also have specific temperature and pressure limits, which directly affect the sterilization methods that can be applied safely.
Key Structural Components of a Medical Balloon Catheter
| Component | Material Type | Function | Sensitivity During Sterilization |
|---|---|---|---|
| Balloon Segment | Polyurethane / Nylon | Expands to dilate or unblock vessels | Sensitive to heat and solvents |
| Catheter Shaft | Polyethylene / Pebax | Provides flexibility and lumen stability | May deform under high temperature |
| Connector Interface | Polycarbonate | Ensures secure connection to control unit | Sensitive to aggressive chemicals |
Understanding these material properties helps define suitable sterilization techniques that do not compromise the mechanical strength or elasticity of the catheter.
The Importance of Sterilization in Balloon Catheter Use
Sterilization is not merely a procedural requirement—it is a safeguard for patient safety and product integrity. As balloon catheters come in direct contact with blood vessels and internal tissues, they must remain free from microbial contamination. Even minimal residues or biofilms can alter the surface energy of the balloon, impairing inflation accuracy or adhesion characteristics.
Additionally, the sterilization process must maintain the catheter’s dimensional precision and burst pressure rating. Excessive heat, chemical exposure, or moisture may weaken material bonds, microscopic cracks or balloon leakage during procedures. Therefore, the sterilization of a medical balloon catheter requires a delicate balance between microbiological safety and mechanical preservation.
Sterilization Methods Suitable for Medical Balloon Catheters
The selection of a sterilization method depends on material compatibility, device configuration, and clinical usage requirements. Among various available techniques, only a few ensure both safety and material preservation.
Ethylene Oxide (EtO) Sterilization
Ethylene oxide sterilization is widely used for thermosensitive medical devices. It effectively eliminates microorganisms at low temperatures while maintaining material flexibility. The process involves controlled humidity, gas concentration, and exposure time. However, residual gas must be carefully aerated before use to prevent patient exposure.
Advantages:
Effective at low temperatures (below 60°C)
Compatible with polymer materials
Penetrates complex catheter lumens
Limitations:
Long aeration time required
Requires precise environmental control
Gamma Radiation Sterilization
Gamma sterilization uses high-energy photons to destroy microbial DNA. It offers uniform penetration and does not require high temperatures. However, prolonged or repeated exposure can cause polymer degradation or discoloration in balloon materials.
Advantages:
Deep penetration and fast process
No chemical residue
Suitable for pre-packaged sterile catheters
Limitations:
May alter mechanical properties over time
Not ideal for repeated sterilization cycles
Hydrogen Peroxide Plasma Sterilization
This modern method uses vaporized hydrogen peroxide under plasma conditions. It ensures effective sterilization without excessive heat or moisture, making it ideal for single-use and reusable catheters alike.
Advantages:
Rapid cycle time
Low temperature exposure
Environmentally safe, with no toxic residues
Limitations:
Limited penetration in long, narrow lumens
Equipment cost and size constraints
Comparison of Common Sterilization Methods
| Method | Temperature Range | Residue Risk | Material Compatibility | Reusability Suitability |
|---|---|---|---|---|
| Ethylene Oxide | 30–60°C | Moderate | Excellent | High |
| Gamma Radiation | Ambient | None | Moderate | Low |
| Hydrogen Peroxide Plasma | 40–55°C | None | Excellent | High |
Maintenance Protocols After Sterilization
Proper maintenance after sterilization ensures that the catheter remains functional until its next use. Maintenance primarily focuses on visual inspection, protective storage, and environmental control.
Inspection and Functional Testing
After sterilization, each medical balloon catheter should undergo thorough inspection to identify potential defects. This includes checking for balloon deformation, lumen blockage, or surface irregularities. Any catheter showing micro-cracks or discoloration should be discarded.
Functional tests may involve verifying balloon inflation symmetry, connector integrity, and shaft flexibility. This ensures that sterilization has not compromised the catheter’s structural or functional stability.
Storage Conditions
Storage plays a crucial role in maintaining catheter sterility and performance. The following conditions are recommended:
Temperature: Maintain between 15°C and 25°C
Humidity: Below 60% relative humidity
Environment: Clean, dry, and free from direct sunlight
Packaging: Use sterile, sealed pouches to prevent dust and microbial ingress
Recommended Maintenance Parameters
| Maintenance Aspect | Recommended Practice | Purpose |
|---|---|---|
| Visual Inspection | Examine balloon, shaft, and connector | Detect deformation or damage |
| Functional Testing | Inflate balloon under controlled pressure | Verify performance consistency |
| Storage Environment | Temperature 15–25°C, humidity <60% | Preserve material elasticity |
| Handling Protocol | Use sterile gloves and avoid sharp tools | Prevent contamination or surface damage |
Handling and Reuse Considerations
While some medical balloon catheters are designed for single use, certain models may support limited reprocessing under strict validation. Reuse decisions must comply with regulatory standards and internal quality assurance protocols.
When reusing a catheter, documentation should track the number of sterilization cycles, material inspection outcomes, and any performance deviation. If the balloon exhibits reduced expansion accuracy or changes in texture, it should be permanently retired.
Furthermore, handling procedures during use must minimize mechanical stress. Overinflation or excessive bending can premature wear, reducing both safety and precision in subsequent applications.
Avoiding Common Sterilization and Maintenance Errors
Several operational errors can compromise the effectiveness of sterilization or shorten the catheter’s lifespan:
Using inappropriate sterilization temperatures: High heat can deform the balloon or soften the shaft.
Inadequate aeration after EtO sterilization: Residual gas may remain trapped within lumens.
Improper drying after cleaning: Moisture promotes microbial regrowth.
Rough handling during inspection: Scratches can micro-leaks during inflation.
A strict procedural checklist should be implemented to mitigate these risks. Regular training for personnel in sterilization handling and catheter maintenance ensures that all devices remain compliant with safety and performance standards.
The Link Between Maintenance Quality and Device Performance
The performance of a medical balloon catheter depends not only on its design but also on the consistency of its maintenance and sterilization protocols. Properly maintained catheters exhibit stable inflation characteristics, accurate pressure response, and consistent flexibility during procedures.
Sterilization, when correctly executed, protects against cross-contamination without altering the balloon’s compliance or burst resistance. Conversely, poor maintenance can cause subtle material fatigue that may balloon rupture or catheter malfunction under clinical stress. Therefore, ongoing quality monitoring and adherence to validated procedures are vital to ensuring the device’s reliability.
Conclusion
The medical balloon catheter is a sophisticated device that demands precise handling throughout its lifecycle. Effective sterilization and maintenance are not isolated steps but integral elements of overall device management. By understanding material sensitivities, choosing appropriate sterilization techniques, and maintaining strict post-process care, medical professionals can preserve catheter integrity, enhance procedural safety, and ensure clinical outcomes.
Proper care of this essential device not only extends its service life but also reinforces confidence in modern minimally invasive interventions—where precision, cleanliness, and reliability are indispensable.
For more information, please call us at +86-18913710126 or email us at [email protected].
Introduction In medical practice, particularly in post-surgical care, body cavity effusion, orthoped...
In the medical field, the safety, stability, and efficiency of fluid transmission are directly relat...
Introduction In modern medical procedures, particularly those involving minimally invasive surgery o...
Vascular interventional procedures are integral to modern cardiovascular medicine, particularly when...
Introduction Single-lumen endobronchial tubes are a critical component of respiratory therapy, espec...
The medical industry is increasingly relying on advanced materials for various applications, and one...